The Qualicon BAX® System method for the detection of Listeria monocytogenes in a variety of food
Background: Traditional quantitative assays for Listeria monocytogenes may require up to 5 days (40-48 hours of incubation) whereas BAX-PCR assays can be completed within 2-3 days (24 hours of incubation) for environmental surfaces swab samples, and product samples.
This qualitative analysis, is highly specific and will detect if only one Listeria monocytogenes cell is present, therefore resulting in a Negative or Positive result. This assay does not tell you how much of the pathogen is present.
Standard sample sizes are 25g. Representative sample portions are collected from sample submitted, mixed with highly selective enrichment media and incubated for 24 to 48 hours. After incubation, cells from the sample enrichment are broken open (lysis) to expose their DNA, which, if the target Listeria monocytogenes DNA is present, will be copied thousands of times, allowing for detection via the BAX Real Time PCR Listeria monocytogenes assay.
If the test is positive, a quantitative analysis (MFHPB-30) must be performed on the same sample to confirm for the presence of the pathogen.
Method: Health Canada (MFLP-28)
Turn Around time: 2-3 Business Days
Reported results: Negative or Positive
SPECIMEN REQUIREMENTS
Specimen Type: Food
Container/Kit: Submit 250 grams of food in a sterile plastic sample bag with round wire closure.
Collection information: See Section Sampling under Food Sampling.
Requisition: Complete all fields of the form. See section Forms under Microbiology Sample Sheet.
Sterile Bag: See section Form under Requisition for Containers and Supplies.
Limitation:None.
Preparation prior to transport: Keep refrigerated or frozen.
Shipping Instructions:
- Ship all foods in containers with hard walls and lids secured in the closed position.
- Shipping containers must be labelled with the submitting organization, unique identifier and contents e.g. FOOD SAMPLES on the outside of the container.
- Shipping containers used for food samples should be dedicated to food samples and not be used for other types of environmental samples.
- Ship dry foods and other shelf stable foods at ambient temperature in a closed container.
- Ship frozen foods in an insulated container with sufficient dry packs to maintain the frozen state.
- Ship perishable foods in an insulated container with sufficient cold packs to maintain a temperature as close to 4°C as possible. If ice is used, contain the ice in a manner that does not allow water contact with the samples.
- Submit all food samples to the laboratory as soon as possible
TEST INFORMATION
Food and environmental swab samples are analyzed by real time polymerase chain reaction (RT-PCR) and/or culture for Listeria monocytogenes:
RT-PCR (MFLP-28)
- RT-PCR will be used in the detection of Listeria monocytogenes from vegetable based products, seafood, dairy products, and environmental swab samples. Listeria monocytogenes culture will be used to confirm RT-PCR positive (Detected) and questionable test results (Refer to Culture), to determine the viability of the organism, and to perform molecular typing on isolates for epidemiological purposes.
Culture (MFHPB-30)
- The presence of Listeria monocytogenes in food is determined by culture techniques, including selective enrichment, followed by isolation on selective agar plates, and biochemical identification.
Listeria monocytogenes should be absent.
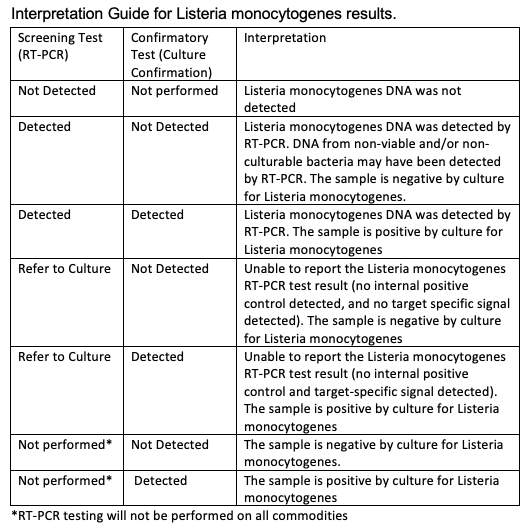
Interpretation of results
For Listeria monocytogenes, the RT-PCR results will be reported as Not Detected, Detected or Refer to Culture – see table below. Confirmation of the viability of the organism will be determined by culture for RT-PCR positive (reported as ‘Detected’) or questionable (reported as ‘Refer to Culture’) test results, as well as all other commodities not verified using the RT-PCR method. Culture confirmation will be reported as Not Detected or Detected.
